The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 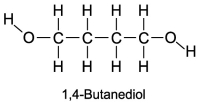
A) Since there are no polar areas on this molecule, it is insoluble in water at room temperature.
B) A high boiling point means that the substance interacts with itself quite strongly. Therefore this molecule is not soluble in water.
C) Since there are polar areas on this molecule, it is insoluble in water at room temperature.
D) Water would be attracted to both ends of 1,4 butanediol, and it is infinitely soluble in water.
Correct Answer:
Verified
Q129: Which of the following intermolecular forces best
Q141: Chlorine, Cl2, is a gas at room
Q142: Two chemical structures are shown, one of
Q142: Why is the surface area of a
Q143: Plastic wrap is made of nonpolar molecules
Q144: How are oxygen molecules attracted to water
Q144: Why are the melting temperatures of most
Q146: Consider the boiling points of the following
Q149: List the following compounds in order of
Q150: Dipole-induced dipole forces of attraction exist between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents