The phosphoric acid salt of caffeine has the structure 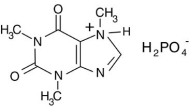 This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?
This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?
A) Salts can't react to form salts, rather they only arise from the reaction of an acid and a base.
B) H3O+H2PO4-
C) Na2+HPO42-
D) Na+H2PO4-
Correct Answer:
Verified
Q57: Why is 2,4,5-trifluorophenol much more acidic than
Q58: Why do ethers have lower boiling points
Q60: Formaldehyde is a toxic preservative with the
Q60: Why might a high-formula-mass alcohol be insoluble
Q61: Rank the following compounds by solubility in
Q63: The amino acid lysine is shown below.
Q64: Alkaloid salts are not very soluble in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents