Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution? 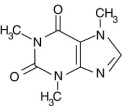
A) A second layer of water would form.
B) Nothing, and the HCl gas would merely bubble out of solution.
C) The diethyl ether insoluble caffeine salt would form as a white precipitate.
D) The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.
Correct Answer:
Verified
Q60: Formaldehyde is a toxic preservative with the
Q60: Why might a high-formula-mass alcohol be insoluble
Q61: Rank the following compounds by solubility in
Q62: The phosphoric acid salt of caffeine has
Q63: The amino acid lysine is shown below.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents