Identify the acid or base behavior of each substance in these reactions: 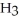
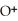 +
+ 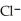 ⇌
⇌ 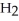 O + HCl
O + HCl
A) 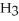
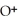 acts as an acid,
acts as an acid, 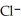 acts as a base,
acts as a base, 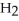 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
B) 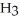
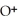 acts as an base,
acts as an base, 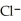 acts as a acid,
acts as a acid, 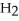 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
C) 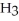
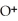 acts as an acid,
acts as an acid, 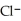 acts as a base,
acts as a base, 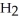 O acts an base, HCl acts as a acid.
O acts an base, HCl acts as a acid.
D) 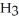
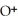 acts as an base,
acts as an base, 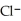 acts as a acid,
acts as a acid, 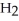 O acts an base, HCl acts as an acid.
O acts an base, HCl acts as an acid.
Correct Answer:
Verified
Q6: For the following acid-base reaction,identify which salt
Q21: What happens to the corrosive properties of
Q24: An acid and a base react to
Q28: Which of the following statements about strong
Q30: What is the main characteristic of a
Q34: Water is formed from the reaction of
Q38: For the following acid-base reaction,identify what is
Q40: What is the main characteristic of a
Q41: Some molecules are able to stabilize a
Q58: Which of the above images would best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents