Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 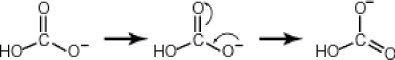 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 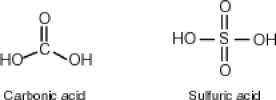
A) In sulfuric acid, the negative charge is able to flip-flop to two additional oxygens rather than only one as is the case for carbonic acid.
B) The two double bonded oxygens in 
 tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
C) Since carbonic acid has resonance stabilization and sulfuric acid does not, sulfuric acid is less stable and more acidic.
D) The acid strength of two comparative molecules is directly proportional to the number of number of oxygens directly bonded to the central atom. Sulfuric acid, having four such oxygens, is more acidic.
Correct Answer:
Verified
Q6: For the following acid-base reaction,identify which salt
Q21: What happens to the corrosive properties of
Q28: Which of the following statements about strong
Q30: What is the main characteristic of a
Q34: Water is formed from the reaction of
Q38: For the following acid-base reaction,identify what is
Q40: Identify the acid or base behavior of
Q40: What is the main characteristic of a
Q46: Does a water molecule become more or
Q58: Which of the above images would best
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents