Does a water molecule become more or less polar when bound to a metal ion, such as 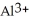 ? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
? Why? (Hint: How might the electrons of the O-H covalent bonds in water "feel" about the highly charged aluminum ion?)
A) Less polar. Aluminum ion is so highly polar that it attracts the electrons in a water molecule to itself and away from the oxygen atom in water, causing water to lose polar character.
B) More polar. The electrons in the O-H bond are drawn even closer to the oxygen (and away from the hydrogen) as they are drawn towards the positive charge of the aluminum ion.
C) Neither. Although polarity may increase as a water molecule approaches an aluminum ion, once the water molecule becomes bound to the metal ion its polarity reverts to its original status.
D) Both. As a first water molecule approaches 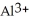 , there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form
, there is an increase in water's polarity. However, the "bond" becomes less polar when the aluminum ion has captured all six water molecules to form 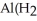 O
O 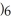 3+.
3+.
Correct Answer:
Verified
Q28: Which of the following statements about strong
Q30: What is the main characteristic of a
Q33: Q40: What is the main characteristic of a Q41: Some molecules are able to stabilize a Q47: How readily an acid donates a hydrogen Q49: Which should be a stronger base: ammonia, Q50: Arrange the following images of an aqueous Q51: Why are aqueous solutions of highly charged Q58: Which of the above images would best![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents