Why are aqueous solutions of highly charged metal ions, such as 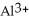 , usually acidic?
, usually acidic?
A) The highly charged 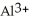 ion strips hydrogen ions away from water as if forms
ion strips hydrogen ions away from water as if forms 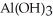 and releases them into the solution causing the solution to become acidic.
and releases them into the solution causing the solution to become acidic.
B) As the water molecule binds to the aluminum ion, the H-O bond becomes more polar. This means that the water molecule is more readily able to form hydrogen ions, which makes the solution acidic.
C) False! 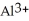 ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
ions tend to make aqueous solutions basic since they pull the OH⁻ ion from the water molecule.
D) The formation of the oxide 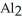
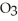 releases hydrogen ions to the aqueous solution making it acidic.
releases hydrogen ions to the aqueous solution making it acidic.
Correct Answer:
Verified
Q29: Which of the following statements about strong
Q33: Q46: Does a water molecule become more or Q46: Can an acid and a base react Q47: How readily an acid donates a hydrogen Q47: Sodium hydroxide, NaOH, is a very strong Q49: Which should be a stronger base: ammonia, Q50: Arrange the following images of an aqueous Q53: Acetic acid, shown below, has 4 hydrogen Q53: Which of the following statements about strong![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents