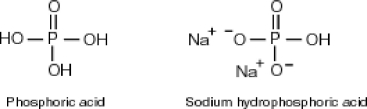
Why is phosphoric acid, 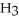
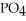 , a stronger acid than disodium hydrogen phosphate,
, a stronger acid than disodium hydrogen phosphate, 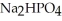 ?
? 
A) Phosphoric acid has three acidic hydrogens which makes it three times as acidic.
B) Some of the released sodium ions in 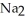 HP
HP 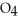 form NaOH (a base) , which decreases the acidity of the
form NaOH (a base) , which decreases the acidity of the 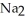 HP
HP 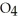 .
.
C) Phosphoric acid dissociates 100% in water whereas 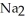 HP
HP 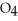 only dissociates about 50%.
only dissociates about 50%.
D) None of the above accurately describes why phosphoric acid is a stronger acid than disodium hydrogen phosphate.
Correct Answer:
Verified
Q44: Would it be easier or harder for
Q46: Can an acid and a base react
Q47: Sodium hydroxide, NaOH, is a very strong
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents