Which is the stronger acid: H-F or H-I?
A) H-F, because the electronegativity of fluorine is higher than that of iodine making the H-F bond more polar and more likely to release the hydrogen ion to solution.
B) H-F, because acid strength for binary acids decreases "down the group" of the periodic table.
C) H-I, because iodine releases the hydrogen in order to form 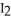 (s) at room temperature.
(s) at room temperature.
D) H-I, because the H-I bond is weaker and so it is easier to break to form hydrogen ions.
Correct Answer:
Verified
Q43: What would the concentration of OH- be
Q44: Would it be easier or harder for
Q47: Q53: Sodium hydroxide,NaOH,is a strong base,which means that Q54: Which of the following statements describes a Q57: A weak acid is added to a![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents