The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 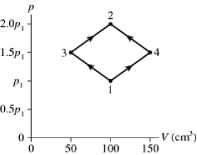
A) (a) 81 atm, (b) 660°C
B) (a) 14 atm, (b) 660°C
C) (a) 81 atm, (b) 120°C
D) (a) 14 atm, (b) 120°C
Correct Answer:
Verified
Q67: If we add 700 J of heat
Q79: A sample of ideal monatomic gas is
Q82: A gas expands from an initial volume
Q208: An ideal gas undergoes the process a→b→c→a
Q209: A rigid container is filled with
Q211: The figure shows a pV diagram
Q215: A sealed 87-
Q216: The figure shows a pV diagram for
Q217: The temperature of an ideal gas
Q218: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents