What is the average kinetic energy of an ideal gas molecule at 569°C? (The Boltzmann constant is 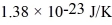 .)
.)
A) 1.74 × 10-20 J
B) 5.81 × 10-21 J
C) 1.18 × 10-17 J
D) 3.93 × 10-19 J
Correct Answer:
Verified
Q44: At what temperature would the root-mean-square speed
Q45: What is the mean free path of
Q46: A sealed container holds 0.020 moles of
Q47: A cubic box with sides of 20.0
Q48: At 50.0°C,the average translational kinetic energy of
Q50: The mean free path of an oxygen
Q51: The mean free path of an oxygen
Q52: What is the root-mean-square value of the
Q53: The root-mean-square speed (thermal speed)of the molecules
Q54: Dust particles are pulverized rock,which has density
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents