The mean free path of an oxygen molecule is 2.0 × 10-5 m,when the gas is at a pressure of 120 Pa and a temperature of 275 K.The molecular mass of oxygen is 32.0 g/mol and the Boltzmann constant is 1.38 × 10-23 J/K,Avogadro's number is 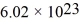 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 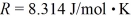 =
= 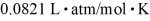 .Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
.Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
A) 20 ns.
B) 43 ns.
C) 95 ns.
D) 200 ns.
E) 420 ns.
Correct Answer:
Verified
Q45: What is the mean free path of
Q46: A sealed container holds 0.020 moles of
Q47: A cubic box with sides of 20.0
Q48: At 50.0°C,the average translational kinetic energy of
Q49: What is the average kinetic energy of
Q51: The mean free path of an oxygen
Q52: What is the root-mean-square value of the
Q53: The root-mean-square speed (thermal speed)of the molecules
Q54: Dust particles are pulverized rock,which has density
Q55: What is the mean free path of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents