Gamma rays are photons with very high energy.How many visible-light photons with a wavelength of 500 nm would you need to match the energy of a gamma-ray photon with energy 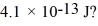 (h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s)
A) 1.0 × 106
B) 1.4 × 108
C) 6.2 × 109
D) 3.9 × 103
Correct Answer:
Verified
Q1: A metal having a work function of
Q3: In a photoelectric effect experiment,electrons emerge from
Q4: If the accuracy in measuring the position
Q5: A light beam from a 2.1-mW He-Ne
Q6: A metal having a work function of
Q7: A metal having a work function of
Q8: Upon being struck by 240-nm photons,a metal
Q9: A photon of initial wavelength 0.651 nm,after
Q10: A stopping potential of 0.50 V is
Q11: When a certain metal is illuminated by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents