A hydrogen atom initially in the n = 6 state decays to the n = 2 state.The emitted photon is detected in a photographic plate.What is the wavelength of the detected photon? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J, 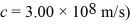
A) 410 nm
B) 93.8 nm
C) 1090 nm
D) 93.1 nm
Correct Answer:
Verified
Q4: If the accuracy in measuring the position
Q10: The energy of the ground state in
Q11: A perfectly black body at 100°C emits
Q12: What is the frequency of the light
Q13: What is the wavelength of peak emission
Q15: What is the orbital radius of the
Q16: Suppose that in a parallel universe,the proton
Q17: Which of the following are characteristics of
Q18: Light excites atomic hydrogen from its lowest
Q19: A hydrogen atom makes a downward transition
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents