A hydrogen atom makes a downward transition from the 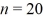 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
A) 2.43 μm
B) 1.46 μm
C) 1.94 μm
D) 2.92 μm
Correct Answer:
Verified
Q14: A hydrogen atom initially in the n
Q15: What is the orbital radius of the
Q16: Suppose that in a parallel universe,the proton
Q17: Which of the following are characteristics of
Q18: Light excites atomic hydrogen from its lowest
Q20: The energy of the ground state in
Q21: An electron inside a hydrogen atom is
Q22: A nonrelativistic proton is confined to a
Q23: A small dust particle of mass 7.90
Q24: Calculate the kinetic energy (in eV)of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents