An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 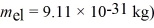
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:
Verified
Q16: Suppose that in a parallel universe,the proton
Q17: Which of the following are characteristics of
Q18: Light excites atomic hydrogen from its lowest
Q19: A hydrogen atom makes a downward transition
Q20: The energy of the ground state in
Q22: A nonrelativistic proton is confined to a
Q23: A small dust particle of mass 7.90
Q24: Calculate the kinetic energy (in eV)of a
Q25: A nonrelativistic electron is confined to a
Q26: A molecule of roughly spherical shape has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents