An alkali metal atom is in the ground state.The orbital angular momentum equals zero and the spin angular momentum is entirely due to the single valence electron.A magnetic field is applied that splits the ground state energy level into two levels,27 μeV apart.What is the strength of the applied magnetic field? (h = 6.626 × 10-34 J ∙ s,Bohr magneton = μB = 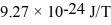 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
A) 0.23 T
B) 0.18 T
C) 0.29 T
D) 0.34 T
E) 0.40 T
Correct Answer:
Verified
Q43: An atom in a state with its
Q44: A three-dimensional potential well has potential U0
Q45: Model a hydrogen atom as a three-dimensional
Q46: The energy of an electron in the
Q47: A three-dimensional potential well has potential U0
Q48: An alkali metal atom is in the
Q49: What is the correct electronic configuration for
Q50: A muonic hydrogen atom is a proton
Q51: An s state (l = 0)energy level
Q52: What is the radius of the smallest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents