Two processes are shown on the pV diagram in the figure.One of them is an adiabat and the other one is an isotherm.Which process is the isotherm? 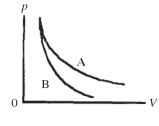
A) process A
B) process B
C) The processes shown are neither isotherms nor adiabats.
D) It is not possible to tell without knowing if the gas is monatomic or diatomic.
Correct Answer:
Verified
Q10: On a cold day,a piece of metal
Q12: The process shown on the pV diagram
Q13: An ideal gas is compressed isobarically to
Q14: A thermally isolated system is made up
Q15: If you wanted to know how much
Q16: Which one of the following quantities is
Q18: The process shown on the TV graph
Q19: By what primary thermal energy transfer mechanism
Q21: It is necessary to determine the
Q22: A cyclic process is carried out on
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents