The figure shows a pV diagram of a gas for a complete cycle.During part bc of the cycle,1190 J of thermal energy through the process of heating flows into a system,and at the same time the system expands against a constant external pressure of as its volume increases from to Calculate the change in internal (thermal)energy of the system during part bc of the cycle.If the change is nonzero,be sure to indicate whether the change is positive or negative. 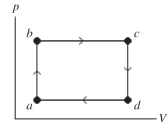
Correct Answer:
Verified
Q77: A 40.0-L container is divided into two
Q110: An external heat source supplies thermal energy
Q111: A gas expands from an initial volume
Q112: In an isochoric process,the internal (thermal)energy of
Q113: The gas in a perfectly insulated
Q114: During an isochoric process,the internal (thermal)energy of
Q115: An athlete doing push-ups performs 650 kJ
Q116: An ideal gas undergoes the process
Q118: During an isothermal process,5.0 J of thermal
Q119: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents