In each of the following diagrams, the x-axis is the reaction coordinate and the y-axis is the potential energy. Which diagram corresponds to a reaction that has an activation energy of 35 kJ and an overall reaction energy of -110 kJ?
A) 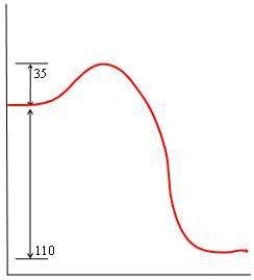
B) 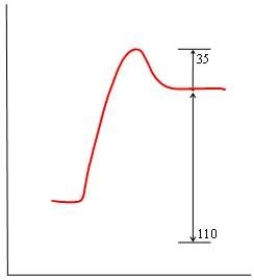
C) 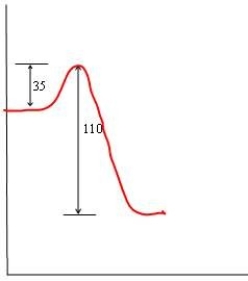
D) 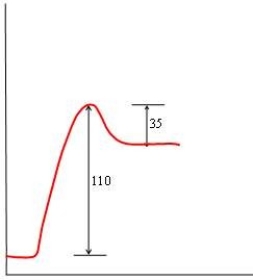
Correct Answer:
Verified
Q55: For an endothermic reaction, the value for
Q56: The reaction: HCl + NaOH → H2O
Q57: Knowing the mechanism of a reaction is
Q59: When the heat of reaction is a
Q61: The magnitude (size)of the energy of activation
Q62: Calculate a value for ΔErxn for a
Q64: The reaction energy profile can tell if
Q80: A lower energy of activation leads to
Q91: It is possible to determine a given
Q98: Reaction rates can be varied by changing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents