Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?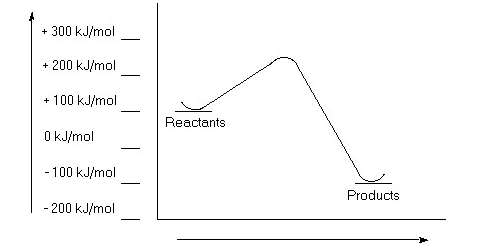
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q57: In each of the following diagrams, the
Q59: When the heat of reaction is a
Q61: The magnitude (size)of the energy of activation
Q64: The value of Ea(reaction)is equal to the
Q67: The reactants must collide with an energy
Q75: When the products are higher in energy
Q80: A lower energy of activation leads to
Q83: The greater the concentration of the reactants,
Q94: Based on the collision theory, adding a
Q98: Reaction rates can be varied by changing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents