The phase diagram for a pure substance is shown below. 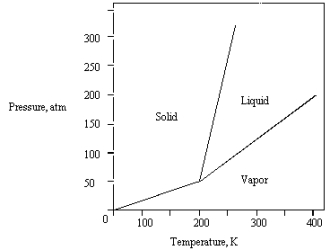 What is the lowest temperature at which liquid can exist?
What is the lowest temperature at which liquid can exist?
A) 400 K
B) 0 K
C) 200 K
D) 150 K
E) 250 K
Correct Answer:
Verified
Q4: A plot of ln(vapor pressure) versus 1/T
Q9: The phase diagram for a pure compound
Q10: Estimate the enthalpy of vaporization of
Q11: The vapor pressure of benzene at
Q11: A plot of ln(vapor pressure) versus 1/T
Q12: Which of the following has the highest
Q15: The vapor pressure of methanol at
Q16: the boiling point of water is higher
Q17: The phase diagram for a pure compound
Q19: In a closed vessel containing water the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents