For the equilibrium CaCO3(s) → CaO(s)+ CO2(g),(a)represents the composition at equilibrium at a certain temperature.In (b),a small amount of Ca*CO3(s)has been added (Ca*CO3(s)represents Ca14CO3(s)or labeled calcium carbonate).Draw the composition in (c)at equilibrium and explain your drawing. 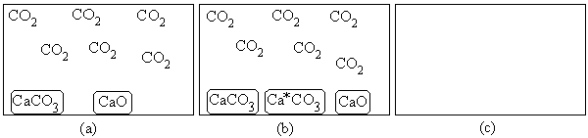
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: Consider the following reaction at a certain
Q10: Consider the reaction
CuSO4(s)
Q11: Consider the reaction
2CuBr2(s)
Q12: For the equilibrium N2O4(g) →2NO2(g),plot,on the same
Q15: Calculate the value of K at 700
Q16: Calculate the equilibrium constant for the
Q18: Consider the reaction
2CuBr2(s)
Q19: What is the relation between K and
Q99: Calculate
Q110: Calculate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents