Use the following diagram of a cell to answer questions 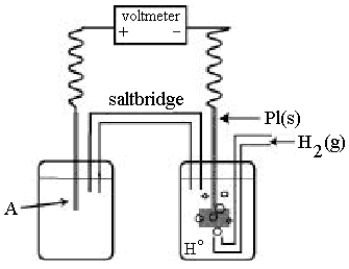
-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which electrode is negative?
Correct Answer:
Verified
Q68: How many moles of Cl2(g)are produced
Q73: In order to convert hydrazine,N2H4,to nitric acid,
A)an
Q74: How many moles of O2(g)are produced by
Q77: Use the following diagram of a
Q244: Use the following diagram of a cell
Q252: How long will it take to deposit
Q253: Of the following metals, which metal would
Q267: When KI(aq)is electrolyzed at a concentration of
Q275: In the Daniell cell,the anode is zinc
Q277: For the reduction of Cu2+ by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents