For the reaction A products,the following data were collected. 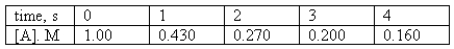
Determine the order of the reaction and calculate the rate constant.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q19: For the reaction 2A + B
Q20: The concentration-time dependence is shown below for
Q20: If the rate of reaction increases by
Q21: Consider the reaction 2N2O5(g)
Q22: The concentration-time curves for two sets of
Q24: For the reaction cyclopropane
Q25: For a given first-order reaction,after 2.00 min,20%
Q26: For a second-order reaction,a straight line is
Q27: The reaction
2ClO2(g)+ F2(g)
Q28: Consider the reaction 2N2O(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents