For the reaction
HO(g) + H2(g) H2O(g) + H(g)
A plot of 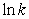 versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
A) 42 kJ.mol-1
B) 98 kJ.mol-1
C) 0.61 kJ.mol-1
D) 5.1 kJ.mol-1
E) 12 kJ.mol-1
Correct Answer:
Verified
Q27: What is the half-life of a reaction
Q42: A catalyst facilitates a reaction by
A) increasing
Q44: The reaction [(CN)5CoOH2]2-(aq)+ SCN-(aq)
Q51: Consider the reaction
NOBr(g)
Q52: An elementary process has an activation energy
Q53: The reaction profile for the reaction
[(CN)5CoOH2]2-(aq)+
Q54: The activation energy of a reaction
Q57: The reaction 2NO(g)+ O2(g)
Q58: For the reaction cyclobutane(g)
Q60: Consider the dimerization reaction below:
2A
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents