Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 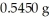 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
A) 7.333%
B) 11.48%
C) 22.95%
D) 45.91%
Correct Answer:
Verified
Q9: Based on the balanced chemical equation shown
Q68: How many milliliters of 0.550 M hydriodic
Q78: If 100.mL of 0.100 M Na2SO4 is
Q144: There are _ mol of bromide ions
Q146: What is the molar concentration of lithium
Q147: Calculate the concentration (M)of sodium ions in
Q151: How many moles of Co2+ are present
Q152: When 15.6 mL of 0.500 M AgNO3
Q153: What is the molar concentration of phosphate
Q154: How many grams of CaCl2 are formed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents