Using the graph below,determine the gas that has the lowest density at STP. 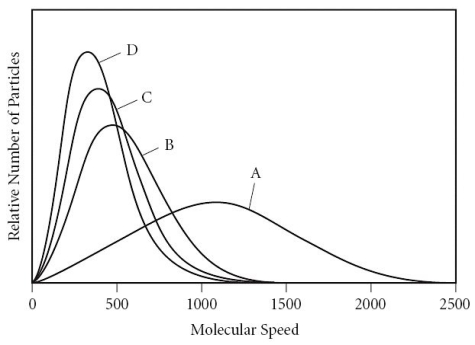
A) A
B) B
C) C
D) D
E) All of the gases have the same density at STP.
Correct Answer:
Verified
Q35: The total pressure of a gas mixture
Q36: A 0.465 g sample of an unknown
Q37: What mass of NO2 is contained in
Q38: A mixture of 10.0 g of Ne
Q39: Using the graph below,determine the gas that
Q41: Determine the mass of water formed when
Q42: The atmospheric pressure is 715 mm Hg.What
Q43: The rate of effusion of two different
Q44: Determine the total volume of all gases
Q45: Which of the gases in the graph
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents