Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat?
A) 10.0 g Ag, CAg = 0.235 J/g°C
B) 10.0 g Pb, 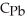 = 0.160 J/g°C
= 0.160 J/g°C
C) 10.0 g water, Cwater = 4.18 J/g°C
D) 10.0 g Fe, CFe = 0.449 J/g°C
E) 10.0 g Ca, CCa= 0.650 J/g°C
Correct Answer:
Verified
Q72: The specific heat capacity of solid copper
Q73: Which of the following (with specific heat
Q74: Which of the following (with specific heat
Q75: Calculate the amount of heat (in kJ)required
Q76: Calculate the change internal energy (ΔE)for a
Q78: A 6.50-g sample of liquid water at
Q79: Give the units of specific heat capacity.
A)
Q80: A balloon is inflated from 0.0100 L
Q81: Identify what a bomb calorimeter measures.
A) measures
Q82: A 50.0-g sample of liquid water at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents