Choose the best Lewis structure for XeI2.
A) 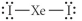
B) 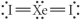
C) 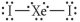
D) 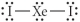
E) 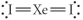
Correct Answer:
Verified
Q26: Identify the number of bonding pairs and
Q27: Define electronegativity.
A) the ability of an atom
Q28: Choose the best Lewis structure for BeF2.
A)
Q29: Give the number of valence electrons for
Q30: Give the number of valence electrons for
Q32: Choose the best Lewis structure for ICl5.
A)
Q33: Give the number of valence electrons for
Q34: Choose the best Lewis structure for SO42⁻.
A)
Q35: Identify the strongest bond.
A) single covalent bond
B)
Q36: Choose the best Lewis structure for NO3⁻.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents