Choose the best Lewis structure for ICl5.
A) 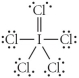
B) 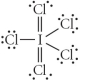
C) 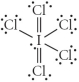
D) 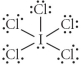
E) 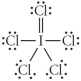
Correct Answer:
Verified
Q27: Define electronegativity.
A) the ability of an atom
Q28: Choose the best Lewis structure for BeF2.
A)
Q29: Give the number of valence electrons for
Q30: Give the number of valence electrons for
Q31: Choose the best Lewis structure for XeI2.
A)
Q33: Give the number of valence electrons for
Q34: Choose the best Lewis structure for SO42⁻.
A)
Q35: Identify the strongest bond.
A) single covalent bond
B)
Q36: Choose the best Lewis structure for NO3⁻.
A)
Q37: Identify the weakest bond.
A) single covalent bond
B)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents