The combustion of ethylene proceeds by the reaction C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
When the rate of disappearance of 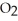 is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
A) 0.15
B) 0.077
C) 0.69
D) 0.35
E) 0.46
Correct Answer:
Verified
Q90: The isomerization of methylisonitrile to acetonitrile CH3NC
Q102: For a particular first-order reaction,it takes 24
Q103: Which of the following represents the equation
Q103: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q104: The reaction that occurs in a Breathalyzer,a
Q105: How many half-lives are required for the
Q106: The rate constant for a second-order reaction
Q108: The elementary reaction 2 NO2(g)→ 2 NO(g)+
Q110: If the concentration of a reactant is
Q111: How many half-lives are required for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents