Derive an expression for a "1/3-life" for a first-order reaction.
A) 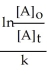
B) 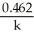
C) 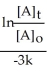
D) 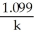
E) 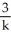
Correct Answer:
Verified
Q53: Nitrogen dioxide decomposes at 300°C via a
Q63: In aqueous solution,hypobromite ion,BrO-,reacts to produce bromate
Q104: Carbon-14,which is present in all living tissue,radioactively
Q107: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q116: The rate constant,k,for a first-order reaction is
Q121: Derive an expression for a "1/4-life" for
Q122: Which rate law is bimolecular?
A) rate =
Q123: Hydrogen iodide decomposes at 800 K via
Q125: Which of the following reactions would you
Q130: For the first-order reaction,2 N2O(g)→ 2 N2(g)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents