For the first-order reaction,2 N2O(g) → 2 N2(g) + O2(g) ,what is the concentration of N2O after 3 half-lives if 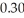 mol of N2O is initially placed into a 1.00-L reaction vessel?
mol of N2O is initially placed into a 1.00-L reaction vessel?
A) 1.9 × 10-2 M
B) 3.8 × 10-2 M
C) 7.5 × 10-2 M
D) 1.5 × 10-1 M
Correct Answer:
Verified
Q53: Nitrogen dioxide decomposes at 300°C via a
Q63: In aqueous solution,hypobromite ion,BrO-,reacts to produce bromate
Q101: The following reaction is first order,C2H6 →
Q107: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q116: The rate constant,k,for a first-order reaction is
Q117: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has
Q125: Which of the following reactions would you
Q126: Derive an expression for a "1/3-life" for
Q133: A particular first-order reaction has a rate
Q135: The isomerization reaction,CH3NC → CH3CN,is first order
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents