A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C.What is the magnitude of k at 95.0°C if Ea = 55.5 kJ/mol?
A) 9.56 × 103 s-1
B) 2.85 × 104 s-1
C) 576 s-1
D) 4.33 × 1087 s-1
E) 1.36 × 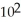 s-1
s-1
Correct Answer:
Verified
Q53: Nitrogen dioxide decomposes at 300°C via a
Q63: In aqueous solution,hypobromite ion,BrO-,reacts to produce bromate
Q101: The following reaction is first order,C2H6 →
Q107: The first-order reaction,SO2Cl2 → SO2 + Cl2,has
Q117: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has
Q130: For the first-order reaction,2 N2O(g)→ 2 N2(g)+
Q135: The isomerization reaction,CH3NC → CH3CN,is first order
Q136: A particular first-order reaction has a rate
Q137: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Q138: Which rate law is termolecular?
A) rate =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents