The Kp for the reaction below is 1.49 × 108 at 100.0°C: CO(g) + 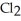 (g) ⇌
(g) ⇌ 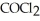 (g)
(g)
In an equilibrium mixture of the three gases, 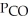 =
= 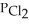 = 2.22 × 10-4 atm.The partial pressure of the product,phosgene (
= 2.22 × 10-4 atm.The partial pressure of the product,phosgene ( 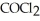 ) ,is ________ atm.
) ,is ________ atm.
A) 7.34
B) 3.02 × 1015
C) 3.31 × 10-16
D) 3.31 × 104
E) 6.67 × 1011
Correct Answer:
Verified
Q79: Express the equilibrium constant for the following
Q80: The equilibrium constant is given for one
Q81: What is Δn for the following equation
Q82: The decomposition of ammonia is: 2 NH3(g)⇌
Q83: The equilibrium constant is given for one
Q85: What is Δn for the following equation
Q86: In which of the following reactions will
Q87: The reaction below has a Kc value
Q88: What is Δn for the following equation
Q89: What is △n for the following equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents