The net ionic equation for the reaction between aqueous ammonia and hydrochloric acid is
A) HCl(aq) + NH3(aq) NH4Cl(aq) .
B) H+(aq) + OH-(aq) H2O( 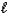 ) .
) .
C) HCl(aq) + OH-(aq) Cl-(aq) + H2O( 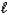 ) .
) .
D) H+(aq) + NH3(aq) NH4+(aq) .
E) H+(aq) + Cl-(aq) + NH3(aq) NH4+(aq) + Cl-(aq) .
Correct Answer:
Verified
Q31: Nitric acid is the product of the
Q55: What are the spectator ions in the
Q56: Metal oxides react with water to produce
Q57: What base results when Li2O reacts with
Q61: Which species in the reaction below
Q63: Which of the following chemical equations
Q64: Write a balanced chemical equation for
Q65: Which species is reduced in the
Q66: A(n)_ agent gains electrons in an oxidation-reduction
Q77: In the reaction of acetic acid with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents