Write a balanced chemical equation for the reaction of aqueous solutions of ammonium sulfate and sodium hydroxide.
A) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH4OH(aq) + SO3(g) + Na2O(aq)
B) (NH3) 2SO4(aq) + NaOH(aq) 2 NH3(g) + NaOHSO4(aq)
C) (NH3) 2SO4(aq) + 2 NaOH(aq) 2 NH3(g) + Na2SO4(aq) + 2 OH-(aq)
D) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH4+(g) + Na2SO4(aq) + 2 OH-(aq)
E) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH3(g) + 2 H2O( 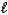 ) + Na2SO4(aq)
) + Na2SO4(aq)
Correct Answer:
Verified
Q31: Nitric acid is the product of the
Q60: The net ionic equation for the
Q61: Which species in the reaction below
Q63: Which of the following chemical equations
Q65: Which species is reduced in the
Q66: A(n)_ agent gains electrons in an oxidation-reduction
Q66: Which of the following are classified
Q67: In the following reaction,which species is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents