The for the reaction of magnesium sulfite with nitric acid is
A) MgSO3(s) + 2H+(aq) Mg2+(aq) + SO2(g) + H2O( 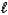 ) .
) .
B) Mg2+(aq) + CO32-(aq) + 2H+(aq) + 2NO3-(aq) Mg(NO3) 2(aq) + SO2(g) + H2O( 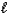 ) .
) .
C) MgSO3(s) + 2HNO2(aq) Mg2+(aq) + 2NO2-(aq) + SO2(g) + H2O( 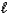 ) .
) .
D) Mg(HSO3) 2(s) + 2HNO3(aq) Mg2+(aq) + 2NO3-(aq) + SO2(g) + 2H2O( 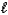 ) .
) .
E) MgSO3(s) + 2HNO3(aq) Mg2+(aq) + 2NO3-(aq) + SO2(g) + H2O( 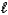 ) .
) .
Correct Answer:
Verified
Q63: Which of the following chemical equations
Q64: Write a balanced chemical equation for
Q65: Which species is reduced in the
Q66: Which of the following are classified
Q67: In the following reaction,which species is
Q69: Which of the following is/are classified
Q70: The net ionic equation for the
Q71: The oxidation number of nitrogen is highest
Q71: _ acid is produced in a larger
Q72: What is the oxidation number of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents