The thermochemical equation for the combustion of methanol is shown below.
CH3OH( 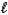 ) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
rH = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g CH3OH?
A) -171 kJ
B) -19.9 kJ
C) -2.38 103 kJ
D) -5.49 103 kJ
E) -1.76 106 kJ
Correct Answer:
Verified
Q26: How much energy is needed to convert
Q27: If 46.1 g Cu at 11.6
Q28: When 66.0 g of an unknown
Q29: A 41.3-g piece of nickel (s =
Q30: Calculate
Q32: Which of the following statements is/are
Q33: Calculate the energy in the form
Q34: Which of the following processes will result
Q35: The thermochemical equation for the combustion
Q37: One statement of the first law of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents