Determine the heat of evaporation of carbon disulfide,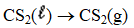
Given the enthalpies of reaction below.
A) -206.1 kJ
B) -27.3 kJ
C) +27.3 kJ
D) +206.1 kJ
E) +1.31 kJ
Correct Answer:
Verified
Q55: Using the following thermochemical data:
Q56: CaO(s)reacts with water to form Ca(OH)2(aq).If
Q57: A bomb calorimeter has a heat
Q58: When 0.236 mol of a weak
Q58: The heat required to convert a solid
Q59: Acetylene,C2H2,is a gas used in welding.The molar
Q61: Dry ice converts directly from a solid
Q63: When 1 mole of Fe2O3(s)reacts with
Q64: The standard enthalpy change for the
Q67: The standard molar enthalpy of formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents