Which of the following reactions corresponds to the thermochemical equation for the standard molar enthalpy of formation of solid calcium nitrate?
A) Ca2+(aq) + 2NO3-(aq) Ca(NO3) 2(s)
B) Ca(OH) 2(s) + 2HNO3(aq) Ca(NO3) 2(s) + 2H2O( 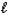 )
)
C) Ca(s) + N2(g) + 3O2(g) Ca(NO3) 2(s)
D) Ca(s) + 2HNO3(aq) Ca(NO3) 2(s) + H2(g)
E) Ca(s) + 2N(g) + 6O(g) Ca(NO3) 2(s)
Correct Answer:
Verified
Q50: In thermodynamics,a(n)_ is defined as the object,or
Q58: The heat required to convert a solid
Q62: Internal energy and enthalpy are state functions.What
Q64: The standard enthalpy change for the
Q66: Calculate
Q69: Which of the following chemical equations
Q71: What is
Q72: Why are you at greater risk
Q73: What is the standard enthalpy of
Q74: Determine the standard enthalpy of formation of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents