How many electrons can be described by the following quantum numbers: n = 5, 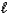 = 0,
= 0, 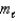 = 0,ms = -1/2?
= 0,ms = -1/2?
A) 1
B) 2
C) 6
D) 10
E) 18
Correct Answer:
Verified
Q6: The maximum number of electrons that can
Q11: The small,but important,energy differences between 3s,3p,and 3d
Q11: Which of the following orbital occupancy
Q14: According to the building-up principle or aufbau
Q15: Which of the following statements concerning ground
Q17: Which element is found in the p-block
Q18: How many electrons can be described by
Q19: Which of the following statements is true
Q20: Which of the following sets of quantum
Q21: What is the ground-state electron configuration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents