What volume of O2,measured at 24.0°C and 0.630 atm atm,will be produced by the decomposition of 3.00 g KClO3? Assume 100% yield.(R = 0.08206 L.atm/mol.K)
2 KClO3(s) 2 KCl(s) + 3 O2(g)
A) 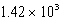 mL
mL
B) 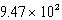 mL
mL
C) 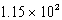 mL
mL
D) 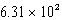 mL
mL
E) none of these
Correct Answer:
Verified
Q51: The density of H2 gas in
Q52: Which of the following gases has
Q53: The density of a gas is 1.96
Q54: Which of the following samples contains the
Q55: What mass of O2 is required
Q57: Ammonia gas is synthesized according to
Q58: A 3.74 gram sample of a certain
Q59: What volume does 40.5 g of N2
Q60: Aqueous hydrochloric acid reacts with magnesium
Q61: What is the volume occupied by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents