What is the distance,in atomic radii,across a diagonal face of a face-centered unit cell?
A) 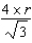
B) 2 r
C) 4 r
D) 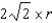
E) r
Correct Answer:
Verified
Q1: If a metal crystallizes in a body-centered
Q7: Polonium (atomic mass 209.0 g/mol)crystallizes in a
Q8: Rhodium crystallizes with a face-centered cubic unit
Q9: In any cubic lattice an atom lying
Q10: Niobium crystallizes in a body-centered cubic lattice.If
Q11: Which of the following statements concerning
Q12: Which of the following statements concerning a
Q15: Gold crystallizes in a face-centered cubic lattice
Q17: Arrange the three common unit cells in
Q18: Which of the following concerning the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents