Henry's Law constant is 0.034 mol/kg.bar and 0.0013 mol/kg.bar for CO2 and O2 respectively at 25 C.What pressure of O2 is required to achieve the same solubility as 0.218 bar of CO2?
A) 5.7 bar
B) 0.0083 bar
C) 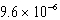 bar
bar
D) 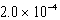 bar
bar
E) 0.18 bar
Correct Answer:
Verified
Q44: Assuming ideal behavior,which of the following
Q45: A solution consisting of 0.228 mol of
Q46: What is the equilibrium partial pressure
Q47: If the solubility of O2 at
Q48: For the following gas-liquid equilibrium for
Q51: What is the freezing point of a
Q52: If a 25.0-g sample of a nonelectrolyte
Q53: What is the boiling-point
Q54: Assuming ideal behavior,which of the following aqueous
Q54: The vapor pressure of water at 90°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents