The Arrhenius equation, 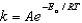 expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
expresses the dependence of the rate constant on the reaction temperature.The slope of a plot of ln(k) versus 1/T is equal to
A) 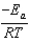
B) 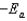
C) 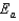
D) 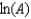
E) 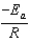
Correct Answer:
Verified
Q37: For which of the following hypothetical rate
Q38: The rate constant of a first-order
Q39: For the reaction A + 2B
Q40: For a certain reaction of the
Q41: The rate constant at 366 K
Q43: For the hypothetical reaction aA
Q44: For a chemical reaction,the activation energy for
Q45: The decomposition of formic acid follows
Q47: Molecules must overcome a barrier called the
Q47: For the formation of 1 mol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents