Given the following chemical equilibrium,
COCl2(g) 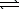
CO(g) + Cl2(g)
Calculate the value of Kc,given that Kp = 6.5 1011 at 298 K.(R = 0.08206 L.atm/mol.K)
A) 1.5 10-12
B) 3.8 10-11
C) 1.1 109
D) 2.7 1010
E) 1.6 1013
Correct Answer:
Verified
Q10: Which expression correctly describes the equilibrium constant
Q11: What balanced equation is the following equilibrium
Q12: Which of the following statements is/are CORRECT?
1)Product
Q13: For which of the following equilibria does
Q14: Write a balanced chemical equation which corresponds
Q16: For which of the following reactions are
Q17: What is the Kc equilibrium-constant expression for
Q18: What is the expression for Kc for
Q19: Write the expression for K for the
Q20: What is the balanced equation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents