The equilibrium constant at 25 C for the dissolution of silver iodide is
8.5 10-17.AgI(s) 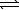 Ag+(aq) + I-(aq)
Ag+(aq) + I-(aq)
If an excess quantity of AgI(s) is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
A) 7.2 10-33 M
B) 4.3 10-17 M
C) 8.5 10-17 M
D) 6.5 10-9 M
E) 9.2 10-9 M
Correct Answer:
Verified
Q5: If the reaction quotient,Q,is equal to K
Q37: When 0.20 mole HF is dissolved
Q38: Consider the following equilibrium: Q39: What is the reaction quotient,Q,for the equilibrium Q40: Nitrogen trifluoride decomposes at to form Q42: The following reaction occurred when a Q43: Consider the following equilibrium: Q44: The equilibrium constant,Kc,for the decomposition of Q45: Nitrogen and oxygen gases may react Q46: For the equilibrium PCl5(g) ![]()
CO2(g)+ H2(g) 
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents