Nitrogen and oxygen gases may react to form nitrogen monoxide.
At 1500 C,Kc equals 1.0 10-5.N2(g) + O2(g) 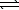 2 NO(g)
2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 C,what is the concentration of NO(g) when equilibrium is established?
A) 3.0 10-7 M
B) 4.7 10-5 M
C) 9.5 10-5 M
D) 3.0 10-2 M
E) 9.1 101 M
Correct Answer:
Verified
Q40: Nitrogen trifluoride decomposes at to form
Q41: The equilibrium constant at 25
Q42: The following reaction occurred when a
Q43: Consider the following equilibrium:
CO2(g)+ H2(g) 
Q44: The equilibrium constant,Kc,for the decomposition of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents